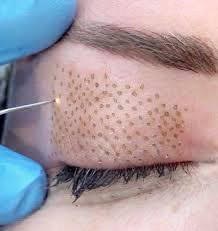
Health Canada is warning Canadians about the potential negative effects of a type of cosmetic device — some of which can likely be found in Orillia, according to a local medical esthetician.
“Health Canada is advising consumers that plasma pens (also known as “fibroblast” devices), promoted for cosmetic skin treatments such as eyelid lifts, wrinkle reduction and removal of moles, skin tags, scars and spots, may pose health risks,” the agency said in a news release. “Plasma pens are small handheld medical devices that focus electricity on the surface of the skin, which causes a controlled burn and spreads heat throughout the targeted area.”
According to Health Canada, potential side effects of plasma pen treatment — “even when the device is used properly — include pain, swelling of the treated area, redness, sagging skin (particularly in the upper eyelids), hyperpigmentation (spots), ultraviolet (UV) sensitivity, and skin peeling and crusting.”
It’s a device Blade Tiessen, who runs the Anti-Aging Clinic and Dispensary in downtown Orillia, has never used on clients.
“There’s too much risk. You’re burning holes in someone’s skin,” he said.
There’s a question about their legality, too, as Health Canada has not approved any of the devices for sale in Canada, “which means that they have not been evaluated for safety, effectiveness or quality,” the release stated.
“Health Canada has been made aware that plasma pens are being used in spas and are also being sold to consumers online and through esthetician training courses.”
Some of the training is inadequate, Tiessen said. He noted one “fibroblast training course” offered a full-day program, yet only 15 minutes was dedicated to learning about skin anatomy, how plasma fibroblast works and what to do post-procedure.
Tiessen, who has been advocating for years for regulation of the industry, said those looking to have procedures done need to do their homework. Someone can receive a certificate after completing minimal training, but that certificate sometimes comes from the manufacturer, not a recognized training institution.
“You need to educate yourselves as to that person’s credentials,” he said.
Health Canada says it is contacting spas and estheticians, as well as provincial and territorial authorities, "to inform them of the medical device licensing requirements and risks associated with the use of plasma pens. The department is also working with the Canada Border Services Agency to help prevent further importation of unauthorized plasma pens."
It urges people to avoid the use of the devices.
To find out if a device has been authorized for sale, check out Health Canada's Medical Devices Active Licence Listing.